AI-Powered Liquid Biopsy Breakthrough Boosts Early Cancer Detection
- Ayusafar ayusafar.health@gmail.com
- Aug 19, 2025
- 1 min read
Saife N. Lone et Sabah Nisar, CC BY 4.0 <https://creativecommons.org/licenses/by/4.0>, via Wikimedia Commons
New Tool Offers Hope for Earlier, More Reliable Cancer Detection
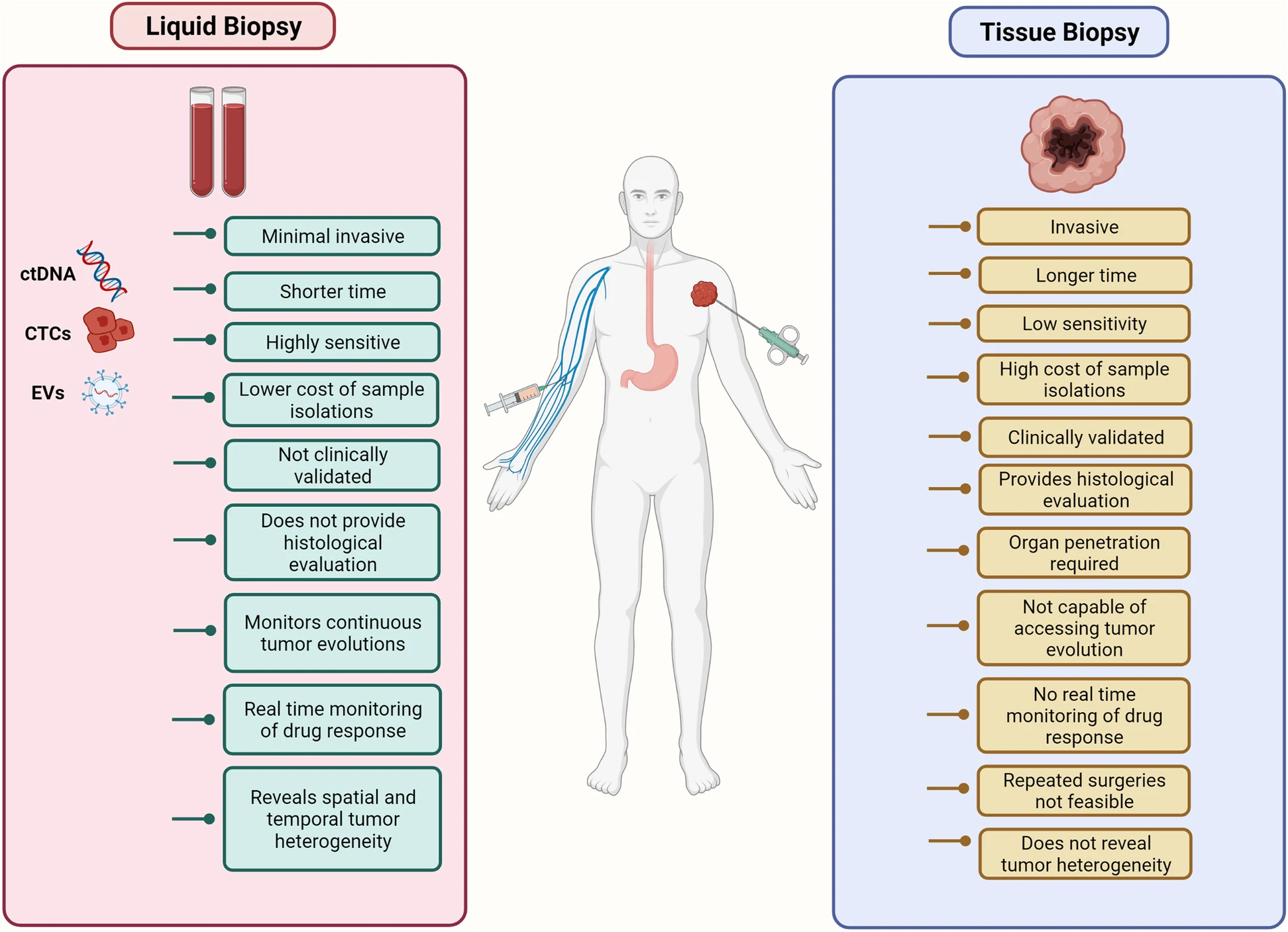
A team at Johns Hopkins University has developed an advanced AI method called MIGHT (Multidimensional Informed Generalized Hypothesis Testing) to dramatically improve the reliability of liquid biopsies—blood tests that detect cancer markers in circulating cell-free DNA (ccfDNA).
Liquid biopsies traditionally struggle with false positives because ccfDNA patterns in autoimmune or vascular disorders can mimic cancer signals. MIGHT addresses this by incorporating diverse datasets into its algorithm—allowing it to distinguish true cancer signals with greater confidence.
Why This Matters
Clinical confidence: Improved specificity means fewer inconclusive results and less anxiety for patients.
Scalable detection: More reliable AI tools can extend liquid biopsy applications across cancer types and settings.
Faster action: Earlier detection means patients can seek treatment sooner, improving outcomes.
Relevance to Indian Patients
In India, especially in cities like Kolkata, many patients rely on local diagnostics before seeking high-cost hospital care. A reliable liquid biopsy could:
Reduce the need for invasive tests like biopsies.
Encourage earlier referral to oncology departments.
Enable faster treatment planning for rural or NRI patients seeking cross-border care.
What Patients & Families Can Do Next
Ask your doctor whether liquid biopsy is available or under trial for your condition.
Watch for broader insurance inclusion, especially under Ayusafar partnerships or government schemes.
Keep healthy records—if you’re at risk or under surveillance, share past data to help algorithms personalize accuracy.
Sources:
Johns Hopkins develops reliable AI for liquid biopsy accuracy ft.com+15News-Medical+15pmc.ncbi.nlm.nih.gov+15
MIGHT method reduces false positives using diverse disease data EurekAlert!+2News-Medical+2




Comments